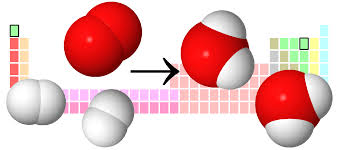
The law of definite proportions states that regardless of the source, chemical compounds are composed of elements combined in fixed ratios. This law is also known as Proust’s Law, named after French chemist Joseph Louis Proust who first proposed the hypothesis. In other words Law of definite proportions says that Atoms combine in definite ratio thus compound have definite proportion of atoms irrespective of the source of the compound. Examples of law of definite proportion: Water,H2O has 11.11% Hydrogen no matter what ever is the source of water.
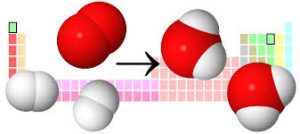
In CO2 , One carbon atom combines with two oxygen atoms so C:O ratio is always 1:2 in CO2 irrespective of the source of origin of CO2. In terms of % of mass, Carbon is 27.27% while Oxygen is 72.72% in CO2 no matter it is exhaled during respiration or it is produced by burning carbon.
Prove the law of constant proportion in the following examples;
Glucose is C6H12O6 . Show that the law of constant proportion is valid in the compound.
Can you write other Examples of law of definite proportion.
Read about law of conservation of mass examples